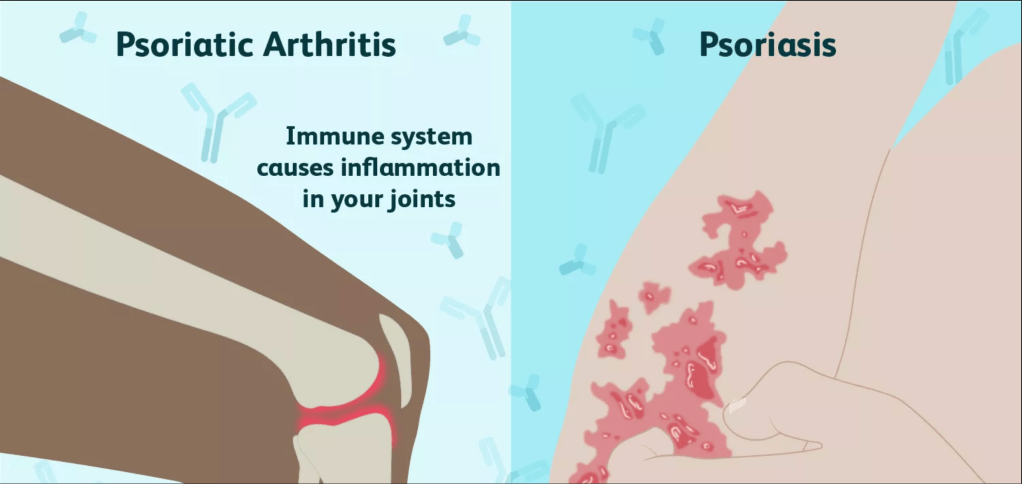
Secukinumab, the drug commercially marketed as Cosentyx, is a monoclonal antibody medication frequently used by sufferers of plaque psoriasis and psoriatic arthritis. Plaque psoriasis is an autoimmune disease affecting the skin that results in raised reddish patches that are covered with whitish buildup of dead skin cells that are often painful and/or itchy. These patches are usually present on the elbows, knees, scalp, or lower back; but they can show up anywhere. T cells and neutrophils in a patient attack healthy skin cells as if they were fighting a wound or infection. These over-acting skin cells can create inflammation in the joints by producing too many new skin cells where there isn’t a need, a condition referred to as psoriatic arthritis. The arthritic condition typically develops after the patches related to psoriasis have appeared, but not always.
Monoclonal antibody drugs like Cosentyx were an answer the experimental issue of our immune systems responding to all of the epitopes of an antigen, resulting in polyclonal antibodies. This is a problem primarily because the antiserum is then different in each time. Monoclonal antibodies are produced by a clone of one type of B cell. Therefore, they attack the same epitope of an antigen. Cosentyx acts as an anti-inflammatory by targeting the cytokine IL-17A, one of the molecules believed to directly affect the inflammation associated with psoriatic disorders. Cosentyx is administered to the patient via injection once a month after the initial weekly shots for four weeks.
Side effects associated with Cosentyx include:
- increased risk of infection
- Nasopharyngitis
- Diarrhea
- Upper respiratory tract infection
- Rhinitis
- Oral herpes
- pharyngitis
- urticaria
- rhinorrhea
Cosentyx works as an anti-inflammatory. This means that it inhibits the innate immune system’s natural responses. While this is useful for the autoimmune condition of psoriasis in that it prevents excessive inflammation in the skin and joints, it also prevents the immune system from attacking actual infections. This mechanism is likely why patients see an increase in upper respiratory tract infections, rhinitis, etc. There are also several drug interactions and adverse reactions to be aware of. In addition to an increased risk of infection by suppressing immune response, the drug also saw many patients in clinical trials experience inflammatory bowl disease and exacerbation of ulcerative colitis. These are inflammatory conditions as well, so it makes sense that Cosentyx would exacerbate them. It is advised that the drug not be administered to patients with an active tuberculosis (TB) infection, and that those with latent TB should be treated prior to starting Cosentyx. The body relies on the immune system to help fight the infection, and Cosentyx negatively impact the innate immune response.
So, is this monoclonal antibody drug a god-send for sufferers like Kim Kardashian West and Art Garfunkel (I know, what a pair)? The answer is unclear. It has given relief to many suffering from psoriasis, but it is important to also consider the side effects of inhibiting IL-17A from binding to the body’s IL-17 receptor–effectively diminishing the innate inflammatory response. What is clear though is that the monoclonal antibody response has been a true breakthrough in developing drug therapy for autoimmune diseases like psoriasis. The hope is of course that research will continue in pursuit of even better drugs with less adverse effects.